
In every situation where equation ( 1) is valid,Įquation ( 3) is valid also-and not vice versa.īoltzmann entropy excludes statistical dependencies That is, equation ( 1) is a corollary ofĮquation ( 3)-and not vice versa. Gibbs gave an explicitly probabilistic interpretation in 1878.īoltzmann himself used an expression equivalent to ( 3) in his later work and recognized it as more general than equation ( 1). He interpreted ρ as a density in phase space-without mentioning probability-but since this satisfies the axiomatic definition of a probability measure we can retrospectively interpret it as a probability anyway. This reduces to equation ( 1) if the probabilities p i are all equal.īoltzmann used a ρ ln ρ formula as early as 1866.
The microstates of such a thermodynamic system are not equally probable-for example, high energy microstates are less probable than low energy microstates for a thermodynamic system kept at a fixed temperature by allowing contact with a heat bath.įor thermodynamic systems where microstates of the system may not have equal probabilities, the appropriate generalization, called the Gibbs entropy, is:
#Entropy definition chemistry plus#
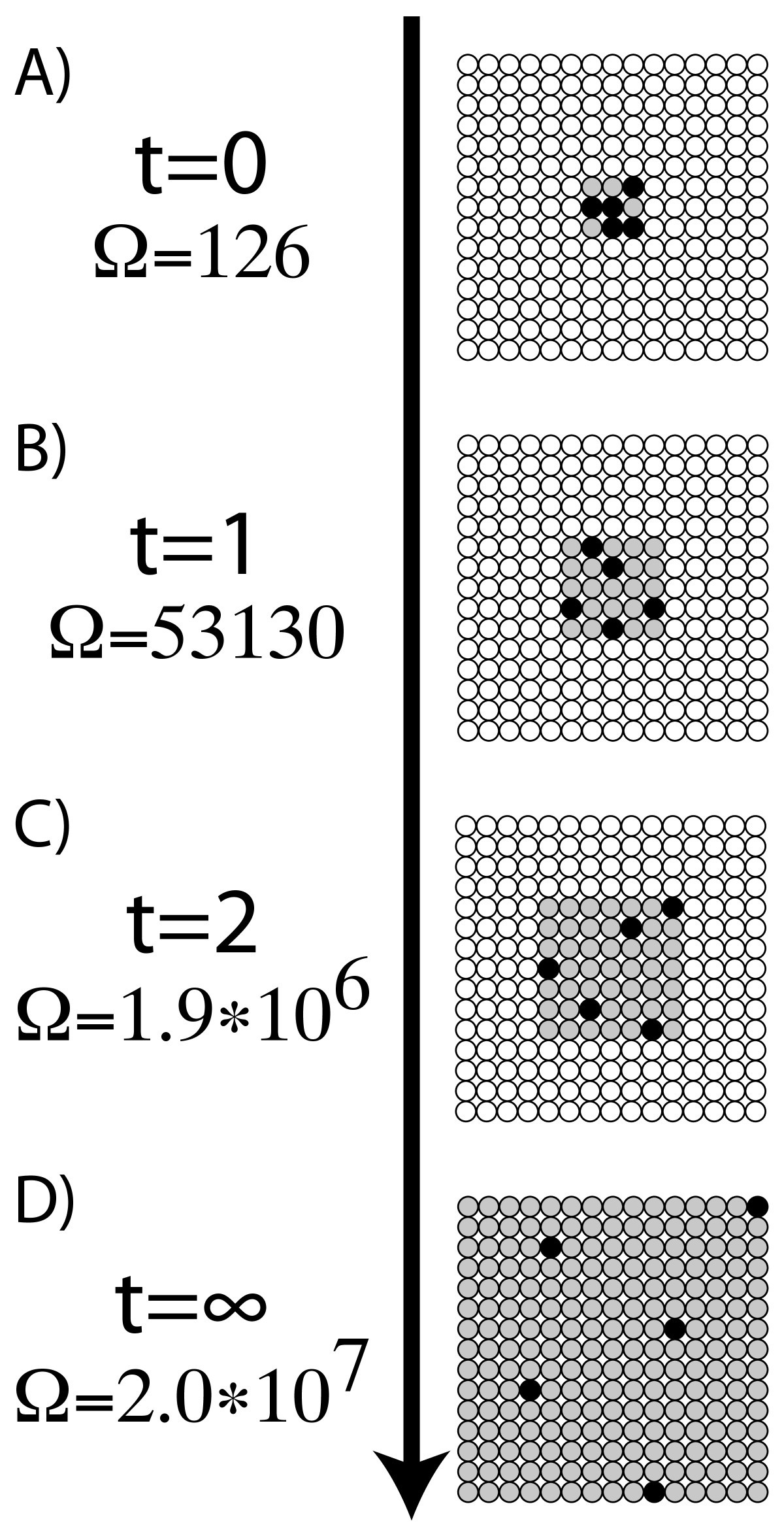
W is sometimes called the "thermodynamic probability" since it is an integer greater than one, while mathematical probabilities are always numbers between zero and one.īoltzmann's formula applies to microstates of a system, each possible microstate of which is presumed to be equally probable.īut in thermodynamics, the universe is divided into a system of interest, plus its surroundings then the entropy of Boltzmann's microscopically specified system can be identified with the system entropy in classical thermodynamics. The "correction" in the denominator is due to the fact that identical particles in the same condition are indistinguishable. Where i ranges over all possible molecular conditions and " !" denotes factorial. W can be counted using the formula for permutations W was historically misinterpreted as literally meaning the number of microstates, and that is what it usually means today. For this case, the probability of each microstate of the system is equal, so it was equivalent for Boltzmann to calculate the number of microstates associated with a macrostate. Boltzmann's paradigm was an ideal gas of N identical particles, of which N i are in the i-th microscopic condition (range) of position and momentum. Interpreted in this way, Boltzmann's formula is the most basic formula for the thermodynamic entropy. Subsequently, Gibbs called it a microcanonical ensemble, and this name is widely used today, perhaps partly because Bohr was more interested in the writings of Gibbs than of Boltzmann.

For single particle instantaneous microstates, Boltzmann called the collection an ergode. For a given macrostate, he called the collection of all possible instantaneous microstates of a certain kind by the name monode, for which Gibbs' term ensemble is used nowadays. Boltzmann considered collections of such microstates. There are many instantaneous microstates that apply to a given macrostate. The value of W was originally intended to be proportional to the Wahrscheinlichkeit (the German word for probability) of a macroscopic state for some probability distribution of possible microstates-the collection of (unobservable microscopic single particle) "ways" in which the (observable macroscopic) thermodynamic state of a system can be realized by assigning different positions and momenta to the respective molecules. The present account concerns instantaneous microstates. In experimental practice, such are scarcely observable. A microstate can be instantaneous, or can be a trajectory composed of a temporal progression of instantaneous microstates. A macrostate is experimentally observable, with at least a finite extent in spacetime.

Ī 'microstate' is a state specified in terms of the constituent particles of a body of matter or radiation that has been specified as a macrostate in terms of such variables as internal energy and pressure. Boltzmann in his kinetic theory of gases". To quote Planck, "the logarithmic connection between entropy and probability was first stated by L. The equation was originally formulated by Ludwig Boltzmann between 18, but later put into its current form by Max Planck in about 1900.
Boltzmann's grave in the Zentralfriedhof, Vienna, with bust and entropy formula.


 0 kommentar(er)
0 kommentar(er)
